Research and Interests
Research Interests:
- Ionic liquids, properties and synthesis
- Asymmetric organic synthesis
- Catalysis
- Molecular modeling
A major problem associated with the use of present homogenous catalysts for asymmetric transformations in organic synthesis is that they are not easily recovered and recycled because they are typically soluble in the reaction media. As a result, large amounts of catalysts are typically used, especially in industry, which poses a disposal problem. In our research, new series of recyclable homogeneous organocatalysts are developed and used to catalyze various asymmetric reactions. These organocatalysts are unique in that they contain ionic liquid moieties, which convey a wide range of properties to the catalysts. As a result, these ionic liquid-supported (ILS) organocatalysts are tunable and their properties can be adjusted to meet a wide variety of reaction conditions. Hence, by changing the polarity of the reaction media after a reaction is complete, the product phase can be separated from the catalyst phase, which makes for easy separation of the catalyst from the products to make the catalyst readily recyclable. Since these catalysts are recyclable and effective in aqueous solvents, they will also provide a “green” alternative and are considered to be eco-friendly organocatalysts.
Ionic Liquid supported catalysts (design and synthesis)
The following are the major advantages of our catalysts: 1) they are stable, 2) they are environmentally friendly, 3) they are recyclable, 4) they require low catalyst loading, and 5) they can be used in aqueous media. For the reactions studied, most give excellent enantio/diastereoselectivities, examples of the catalysts that have been designed and synthesized in our lab are shown in Figure 1.
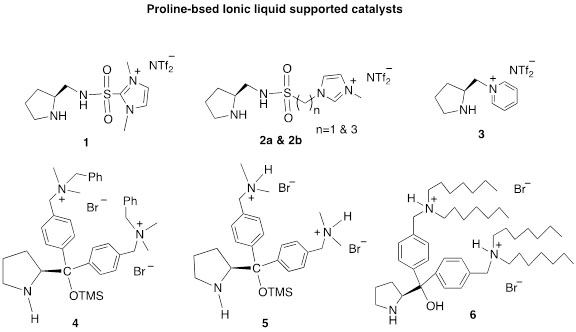
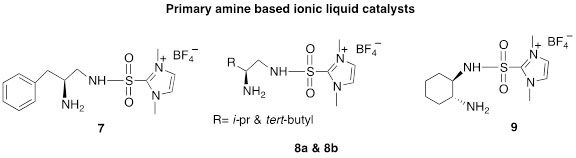
Figure 1. Ionic liquid supported catalysts developed and synthesized in our lab.
Applications of ILS catalysts to key carbon-carbon asymmetric reactions
There are two important carbon-carbon forming reactions that our group has studied utilizing these catalysts: 1) the aldol asymmetric reaction and 2) the Michael asymmetric reaction.
1. Aldol Asymmetric Reactions
For these very important carbon-carbon forming reactions, we have demonstrated that low catalyst loading is required and the reactions can be carried out in aqueous conditions. In addition, the catalyst can be recycled several times with only slight reduction in the stereochemical outcome of the reactions. Below is an example of one of the reactions that we have investigated using various catalysts shown in Figure 1 (Qiao,Y.; Chen, Q.; Lin, S.; Ni, B.; Hadley, A. D., J. Org. Chem 2013, (in press).
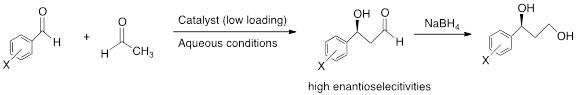
Scheme 2. Example of aldol reaction.
A practical application of the aldol reaction in which our catalysts are used is for the key step in the total synthesis of (R)-convolutamydine A, which exhibits a potent inhibitory activity towards the differentiation of HL-60 human promyelocytic leukemia cells (Scheme 3).
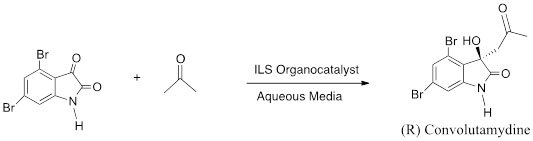
Scheme 3. Key step in the total synthesis of (R)-convolutamydine A.
For this reaction, we have developed a model to study the various factors that influence the outcome of this type reaction (Scheme 3).
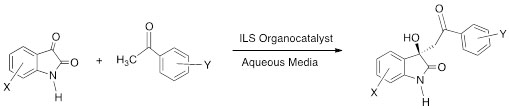
Scheme 3. Model reaction to study the key step in the synthesis of (R)-Convolutamydine.
These reactions are presently being investigated in our lab to determine the optimum reaction conditions for this type reaction using the various catalysts shown in Figure 1.
2. Michael Asymmetric Reactions
The Michael reaction is one of the most powerful methods for the synthesis of new carbon-carbon bonds in which the new compounds are synthetically useful for the synthesis of other compounds. The organocatalysts developed in our lab have been used to successfully catalyze a wide range of asymmetric Michael reactions (Figure 4).

Scheme 4. Example of Michael reaction
A particular reaction is presently being investigated utilizing our catalysts, and it is the key step in the synthesis of Tamiflu (Oseltamivir), which is an antiviral drug for the treatment of influenza A and B (Scheme 5).
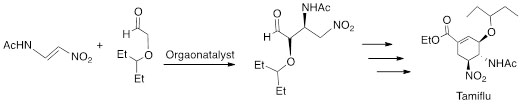
Scheme 5. Key step in the synthesis of Tamiflu (Oseltamivir).
For this reaction, we have developed a model to study the various factors that influence the outcome of this type reaction, which is shown in Scheme 6.
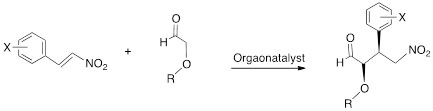
Scheme 6. Model reaction for the key step step in the synthesis of Tamiflu (Oseltamivir).
These reactions are presently being investigated in our lab to determine the optimum reaction conditions for this type reaction using the various catalysts shown in Figure 1.
3. Theoretical Analysis of Transition states for Reactions
In our lab, Spartan is used to assist in studying the nature of the transition states of the reactions of our research. The transition states for the various carbon-carbon forming reactions using our catalysts are located with density functional theory (DFT) with the B3LYP/6-31G(d,p) function and basis set. For example, the transition state for the aldol reaction involving acetone and benzaldehyde using proline as a catalyst is shown in Figure 2.
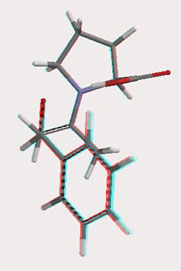
Figure 2. Transition state for the reaction of benzaldehyde and acetone with proline as the catalyst, calculated at using B3LYP/6-31G(d,p).


